Latest Headlines
CATEGORY
-
 2024-04-09Biosyngen reached a significant milestone in the Clinical Trial for BRG01, all patients for Phase 1 have completed dosing in less than a yearBiosyngen, an emerging biotech in the domain of immune cell therapy, pioneering multiple first-in-class products tailored for the treatment of various cancers such as nasopharyngeal cancer, liver cancer, digestive tract tumors, lung cancer, and other solid tumors. The company has secured eight cliniREAD MORE
2024-04-09Biosyngen reached a significant milestone in the Clinical Trial for BRG01, all patients for Phase 1 have completed dosing in less than a yearBiosyngen, an emerging biotech in the domain of immune cell therapy, pioneering multiple first-in-class products tailored for the treatment of various cancers such as nasopharyngeal cancer, liver cancer, digestive tract tumors, lung cancer, and other solid tumors. The company has secured eight cliniREAD MORE -
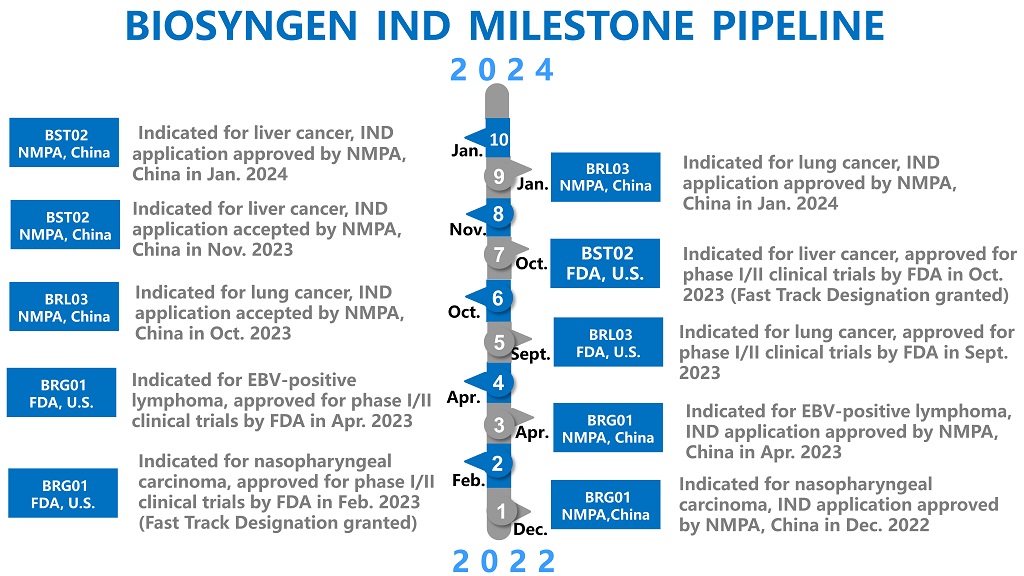
 2024-02-01Biosyngen Announces FDA Fast Track Designation for BST02 in Treatment of Liver CancerFebruary 1, 2024 - Biosyngen is proud to announce that its latest groundbreaking product, BST02, has been granted Fast Track Designation (FTD) by the U.S. Food and Drug Administration (FDA) for the treatment of all types of liver cancer, including hepatocellular carcinoma and cholangiocarcinoma.READ MORE
2024-02-01Biosyngen Announces FDA Fast Track Designation for BST02 in Treatment of Liver CancerFebruary 1, 2024 - Biosyngen is proud to announce that its latest groundbreaking product, BST02, has been granted Fast Track Designation (FTD) by the U.S. Food and Drug Administration (FDA) for the treatment of all types of liver cancer, including hepatocellular carcinoma and cholangiocarcinoma.READ MORE -
 2024-01-24Biosyngen was granted an IND approval for its BST02, the world’s first TIL cell therapy for liver cancer to enter clinical trialOn January 23, 2024, Biosyngen announced that its independently developed TIL therapy BST02 has been granted an IND approval by the Center for Drug Evaluation (CDE), NMPA, China for the treatment of all types of liver cancer.READ MORE
2024-01-24Biosyngen was granted an IND approval for its BST02, the world’s first TIL cell therapy for liver cancer to enter clinical trialOn January 23, 2024, Biosyngen announced that its independently developed TIL therapy BST02 has been granted an IND approval by the Center for Drug Evaluation (CDE), NMPA, China for the treatment of all types of liver cancer.READ MORE -
 2024-01-16Biosyngen obtained IND approval from the NMPA China for its BRL03, a fourth-generation TCR therapy indicated for various solid tumors, including lung cancerEndorsed by Center for Drug Evaluation (CDE), NMPA, on January 15, 2024, Biosyngen’s third global exclusive product pipeline BRL03 was granted an IND approval for the treatment of advanced solid tumors, against lung cancer and gastric cancer.READ MORE
2024-01-16Biosyngen obtained IND approval from the NMPA China for its BRL03, a fourth-generation TCR therapy indicated for various solid tumors, including lung cancerEndorsed by Center for Drug Evaluation (CDE), NMPA, on January 15, 2024, Biosyngen’s third global exclusive product pipeline BRL03 was granted an IND approval for the treatment of advanced solid tumors, against lung cancer and gastric cancer.READ MORE -
 2023-10-27Biosyngen's BST02, the World's First TIL Therapy for Liver Cancer, is Granted an IND Approval by FDAOn October 26, 2023, Biosyngen’s TIL therapy BST02 for liver cancer was granted an approval for clinical trial by the US FDA. BST02, a breakthrough product in the field of cell and gene therapy, represents the world's first TIL therapy designed for the treatment of all types of liver cancer to progrREAD MORE
2023-10-27Biosyngen's BST02, the World's First TIL Therapy for Liver Cancer, is Granted an IND Approval by FDAOn October 26, 2023, Biosyngen’s TIL therapy BST02 for liver cancer was granted an approval for clinical trial by the US FDA. BST02, a breakthrough product in the field of cell and gene therapy, represents the world's first TIL therapy designed for the treatment of all types of liver cancer to progrREAD MORE -
 2023-09-18Biosyngen received the Asia Pacific Cell & Gene Therapy Excellence Awards (APCGTEA) 2023On September 14, 2023, Biosyngen Pte Ltd (hereinafter referred to as "Biosyngen") was the recipient of the prestigious "Most Promising Cell Therapy Pipeline in APAC" award at the 7th Annual Cell & Gene Therapy World Asia 2023, amidst a pool of international biotechnology companies.READ MORE
2023-09-18Biosyngen received the Asia Pacific Cell & Gene Therapy Excellence Awards (APCGTEA) 2023On September 14, 2023, Biosyngen Pte Ltd (hereinafter referred to as "Biosyngen") was the recipient of the prestigious "Most Promising Cell Therapy Pipeline in APAC" award at the 7th Annual Cell & Gene Therapy World Asia 2023, amidst a pool of international biotechnology companies.READ MORE -
 2023-09-11Biosyngen received FDA approval for Phase I/II Clinical Trials for BRL03, targeting Lung Cancer, Gastric Cancer and other advanced Solid TumorsOn September 9th, 2023, Biosyngen Pte Ltd (hereafter as “Biosyngen”) announced that the U.S. FDA has cleared the Investigational New Drug (IND) application for Phase I/II clinical trial of BRL03 for the treatment of lung cancer, gastric cancer and other advanced solid tumors. The approval granted toREAD MORE
2023-09-11Biosyngen received FDA approval for Phase I/II Clinical Trials for BRL03, targeting Lung Cancer, Gastric Cancer and other advanced Solid TumorsOn September 9th, 2023, Biosyngen Pte Ltd (hereafter as “Biosyngen”) announced that the U.S. FDA has cleared the Investigational New Drug (IND) application for Phase I/II clinical trial of BRL03 for the treatment of lung cancer, gastric cancer and other advanced solid tumors. The approval granted toREAD MORE -
 2023-06-05Biosyngen’s Cell Therapy BRG01 Granted Orphan Drug Designation by the U.S. FDA for Treatment of Nasopharyngeal CancerJun. 1st, 2023, Biosyngen Pte. Ltd. (hereinafter as “Biosyngen”) announced that the U.S. Food and Drug Administration’s (FDA) Office of Orphan Products Development (OOPD) has granted to its application, for immune cell therapy BRG01for the treatment of nasopharyngeal cancer, the status of Orphan DruREAD MORE
2023-06-05Biosyngen’s Cell Therapy BRG01 Granted Orphan Drug Designation by the U.S. FDA for Treatment of Nasopharyngeal CancerJun. 1st, 2023, Biosyngen Pte. Ltd. (hereinafter as “Biosyngen”) announced that the U.S. Food and Drug Administration’s (FDA) Office of Orphan Products Development (OOPD) has granted to its application, for immune cell therapy BRG01for the treatment of nasopharyngeal cancer, the status of Orphan DruREAD MORE -
 2023-04-19Biosyngen announces FDA IND approval of its second product for EBV-positive lymphomaApril 15, 2023, Biosyngen Pte Ltd (hereinafter as “Biosyngen”) announced that the Company received IND approval for its second product in the pipeline, a T-cell redirection therapy for the treatment of EBV-positive lymphoma. A week prior to this, the IND application of the same therapy has just beenREAD MORE
2023-04-19Biosyngen announces FDA IND approval of its second product for EBV-positive lymphomaApril 15, 2023, Biosyngen Pte Ltd (hereinafter as “Biosyngen”) announced that the Company received IND approval for its second product in the pipeline, a T-cell redirection therapy for the treatment of EBV-positive lymphoma. A week prior to this, the IND application of the same therapy has just beenREAD MORE -
 2023-04-11Biosyngen received China NMPA IND approval for its T-cell redirection therapy targeting EBV-positive LymphomaApril 7th, 2023, Biosyngen Pte Ltd (hereinafter as “Biosyngen”) was granted IND approval by China NMPA for the company’s first-in-class T-cell redirection therapy, it is an autologous T cell therapy for EBV-positive lymphoma. The principle of autologous T cell therapy is to genetically modify patienREAD MORE
2023-04-11Biosyngen received China NMPA IND approval for its T-cell redirection therapy targeting EBV-positive LymphomaApril 7th, 2023, Biosyngen Pte Ltd (hereinafter as “Biosyngen”) was granted IND approval by China NMPA for the company’s first-in-class T-cell redirection therapy, it is an autologous T cell therapy for EBV-positive lymphoma. The principle of autologous T cell therapy is to genetically modify patienREAD MORE



