Biosyngen reached a significant milestone in the Clinical Trial for BRG01, all patients for Phase 1 have completed dosing in less than a year
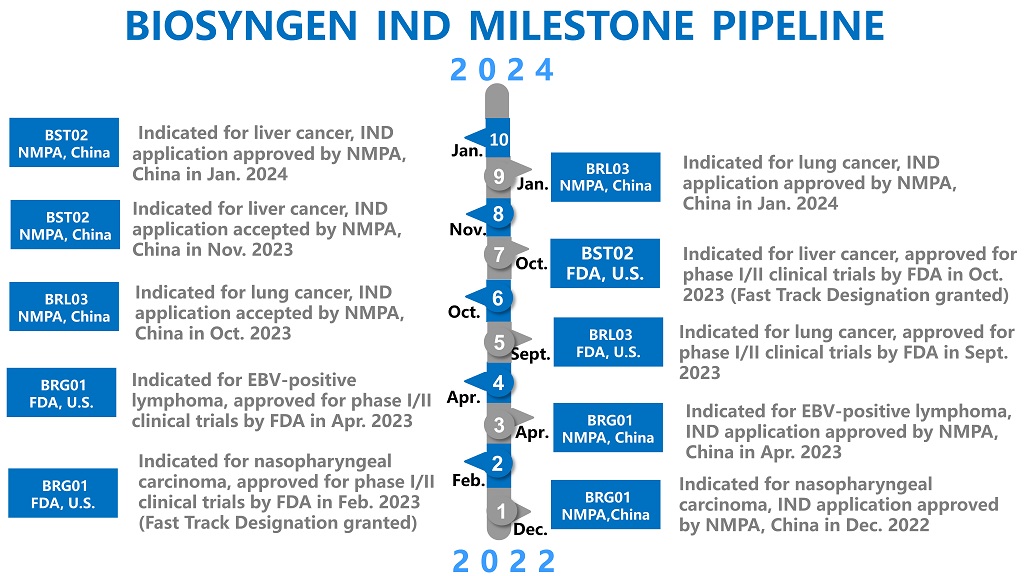
Biosyngen, an emerging biotech in the domain of immune cell therapy, pioneering multiple first-in-class products tailored for the treatment of various cancers such as nasopharyngeal cancer, liver cancer, digestive tract tumors, lung cancer, and other solid tumors. The company has secured eight clinical trial approvals in the United States and China, distinguishing itself as one of few biotechnology firms capable of concurrently applying different technologies in T-cell therapies for in-human trials – namely CAR-T, TCR-T, and TIL.
BRG01, a CAR-T therapy designed to target the Epstein-Barr virus (EBV) antigen, has successfully completed its Phase I clinical trial enrollment at the end of January 2024 following a thorough evaluation process. Within less than one year, all subjects have received treatment with BRG01, exhibiting favorable safety profiles and promising initial efficacy results. These outcomes have established a robust groundwork for the forthcoming Phase II clinical trial scheduled for this year. Concurrently, Phase I clinical trials for two other pioneering products, BST02 and BRL03, are also ongoing. BST02, a novel TIL therapy, exhibits promising potential for liver cancer treatment, while BRL03, a next-generation TCR-T therapy, is geared towards addressing a spectrum of solid tumors, with anticipated advancements in its clinical progress. Both the TIL and TCR-T products are expected to conclude Phase I clinical trials within the current year.
Biosyngen made grounds in innovative drug discovery owing to its persistent endeavors and notable capabilities in-house. The company boasts an experienced research and development team, along with state-of-the-art facilities, encompassing research and development capabilities from drug discovery to clinical application. The company is committed to augmenting investments in research and development, ushering more innovative drugs into clinical trials, and bringing enhanced treatment alternatives for patients.
The ethos of "Limited resources, unlimited possibilities" epitomizes the company's corporate vision, moving forward with confidence and optimism to play a part in the future landscape of the pharmaceutical industry.