Biosyngen was granted an IND approval for its BST02, the world’s first TIL cell therapy for liver cancer to enter clinical trial
On January 23, 2024, Biosyngen announced that its independently developed TIL therapy BST02 has been granted an IND approval by the Center for Drug Evaluation (CDE), NMPA, China for the treatment of all types of liver cancer.
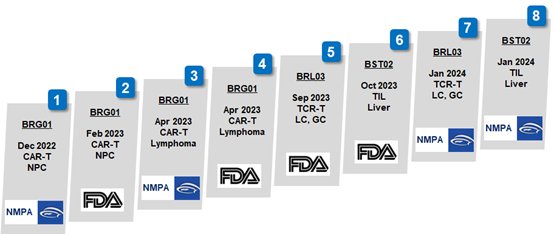
This marks another significant development following FDA’s approval for BST02 in October last year. As the eighth IND approval granted to Biosyngen’s first-in-class products, it underscores the company's prominent role in innovative drug development. BST02 represents the world's first TIL cell therapy for liver cancer to progress to the clinical trial, thereby bolstering the company's competitive standing in the industry.
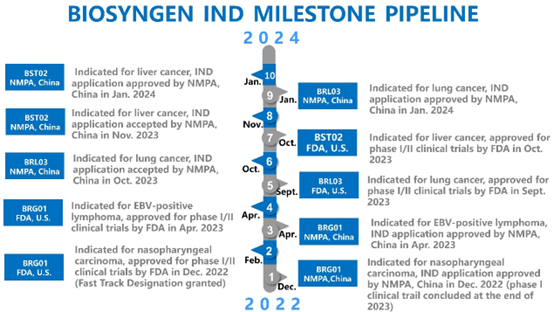
Professor Jean-Paul Thiery, the Chief Scientist and Chairman of the Scientific Committee at Biosyngen, stated that this IND approval by NMPA represents a significant achievement for Biosyngen. This marks the introduction of the company’s all four pioneering pipelines, covering the three prominent T cell therapies of CAR-T, TCR-T, and TIL, into the clinical stage. Furthermore, it is noteworthy that two of these products are on the verge of entering Phase II clinical trials, taking Biosyngen a step closer to addressing an unmet clinical need and bridging a gap in the market.
Tumor-infiltrating lymphocyte (TIL) therapy involves the collection of infiltrating lymphocytes from tumors, followed by their in vitro culture and amplification, and subsequent infusion back into patients for treatment. The distinct T cell receptor (TCR) clonality, enhanced tumor-targeting capability, and reduced off-target toxicity of TIL therapy confer it with distinct advantages in the treatment of solid tumors when compared to other forms of adoptive cellular therapy.
Liver cancer, encompassing hepatocellular carcinoma and intrahepatic cholangiocarcinoma, is a prevalent form of malignant tumor globally. According to GLOBOCAN2020, approximately 906,000 individuals are diagnosed with liver cancer annually, with 830,000 succumbing to the disease. In China, the annual incidence of liver cancer is estimated at 410,000 cases, resulting in approximately 391,000 fatalities. Consequently, there is an imperative demand for effective liver cancer treatment.
BST02, a T cell therapy based on the expansion of the patient's own tumor infiltrating lymphocytes, falls within the category of adoptive immune cell therapy technology. It holds promise for the treatment of all types of liver cancer, offering new hope for patients. In contrast to traditional TIL therapies, BST02 offers numerous benefits, including the ability to overcome distance constraints due to its cryopreserved form and the reduced need for high doses of interleukin-2. Primary clinical trials have demonstrated favorable safety and efficacy outcomes for BST02.
Biosyngen is committed to enhancing the safety and effectiveness of therapeutic interventions for patients. The approval of the Investigational New Drug (IND) application for BST02 signifies the affirmation of the company's research and development competencies and offers substantial impetus for its prospective expansion. Through its accomplishments in cell therapy, Biosyngen continues to uphold its dedication to addressing unmet medical requirements and promoting progress in human health.


