Biosyngen obtained IND approval from the NMPA China for its BRL03, a fourth-generation TCR therapy indicated for various solid tumors, including lung cancer
Endorsed by Center for Drug Evaluation (CDE), NMPA, on January 15, 2024, Biosyngen’s third global exclusive product pipeline BRL03 was granted an IND approval for the treatment of advanced solid tumors, against lung cancer and gastric cancer.
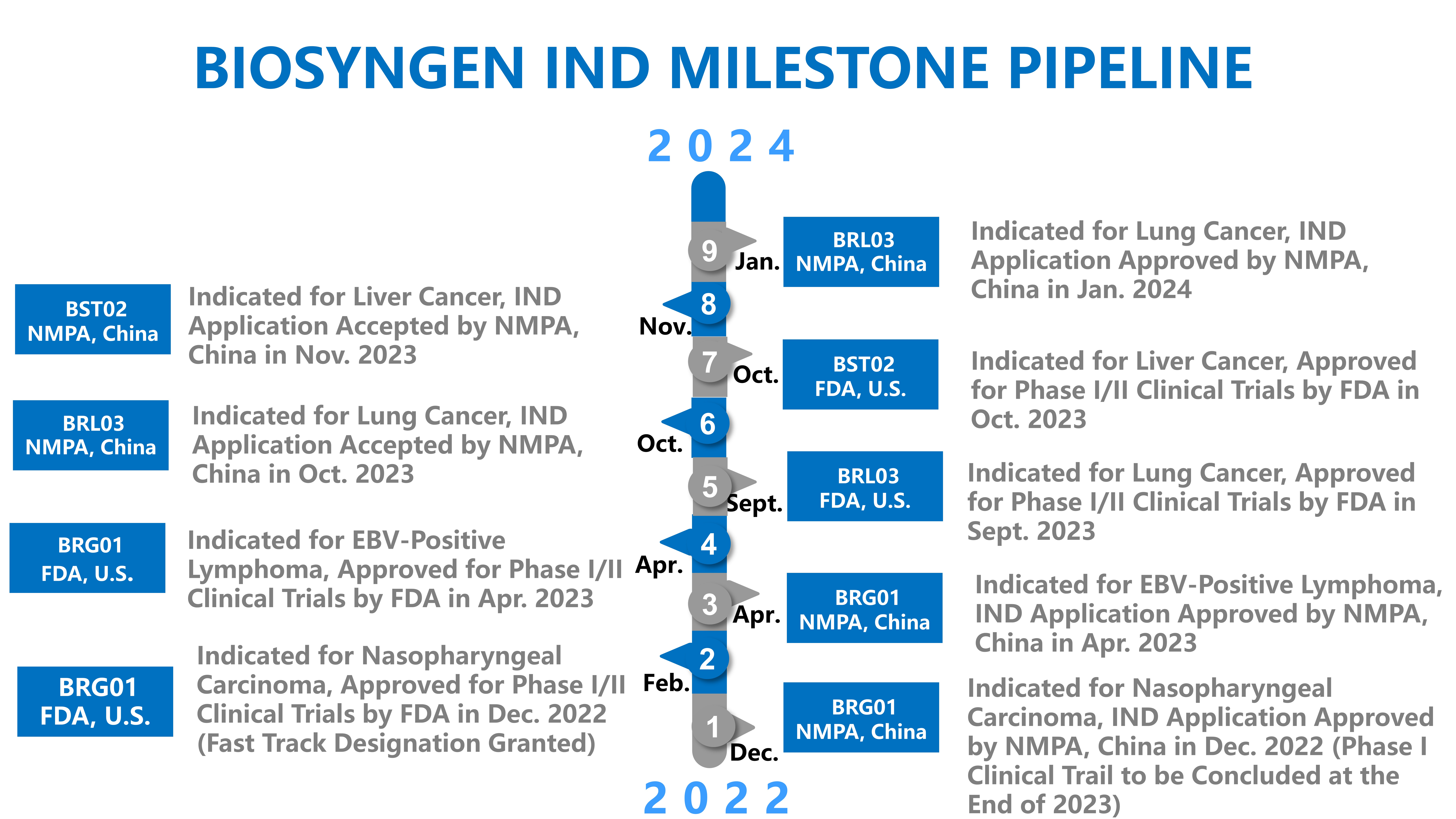
The US FDA has also approved the BRL03 phase I/II clinical trial application on September 9 of the previous year. The NMPA IND approval granted for BRL03 represents the seventh IND approval for the company’s first-in-class products pipelines. As the first TCR-T product independently developed by Biosyngen to enter the clinical stage, the IIT study has demonstrated good safety and primary efficacy profile of BRL03 in the treatment of solid tumors.
![]()
Furthermore, Biosyngen’s two product for nasopharyngeal cancer and EBV positive lymphoma have entered the phase I clinical trial globally, with the nasopharyngeal cancer indication having completed subject enrollment.
Biosyngen’s indigenous innovation technology platform, IDENTIFIER®, plays a crucial role in the discovery and identification of antigens, antibodies, and TCRs. Leveraging a TCR and tumor proteome database from thousands of individuals, the company can efficiently screen and optimize development assets for various therapeutic needs within two weeks. Through this platform, Bisoyngen can screen and select TCRs with high specificity and affinity for a wide range of solid tumors, catering to a broad audience. Additionally, based on another self-developed MSE-T platform, BRL03 also incorporates an additional functional module component that makes T cells less susceptible to depletion in the solid tumor microenvironment, thereby achieving a more durable tumor suppressing effect.
Biosyngen remains dedicated to addressing unmet clinical needs and offering optimal treatment options for cancer patients worldwide.


