Latest Headlines
CATEGORY
-
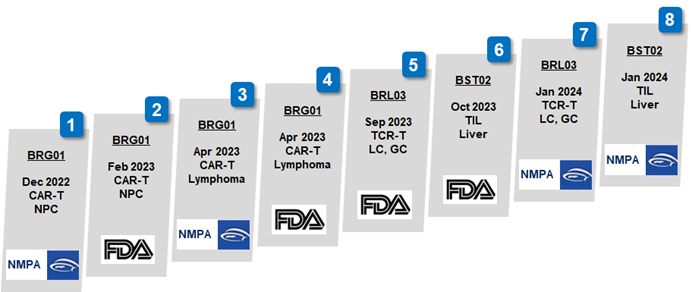 2024-01-24Biosyngen was granted an IND approval for its BST02, the world’s first TIL cell therapy for liver cancer to enter clinical trialOn January 23, 2024, Biosyngen announced that its independently developed TIL therapy BST02 has been granted an IND approval by the Center for Drug Evaluation (CDE), NMPA, China for the treatment of all types of liver cancer.READ MORE
2024-01-24Biosyngen was granted an IND approval for its BST02, the world’s first TIL cell therapy for liver cancer to enter clinical trialOn January 23, 2024, Biosyngen announced that its independently developed TIL therapy BST02 has been granted an IND approval by the Center for Drug Evaluation (CDE), NMPA, China for the treatment of all types of liver cancer.READ MORE -
 2024-01-16Biosyngen obtained IND approval from the NMPA China for its BRL03, a fourth-generation TCR therapy indicated for various solid tumors, including lung cancerEndorsed by Center for Drug Evaluation (CDE), NMPA, on January 15, 2024, Biosyngen’s third global exclusive product pipeline BRL03 was granted an IND approval for the treatment of advanced solid tumors, against lung cancer and gastric cancer.READ MORE
2024-01-16Biosyngen obtained IND approval from the NMPA China for its BRL03, a fourth-generation TCR therapy indicated for various solid tumors, including lung cancerEndorsed by Center for Drug Evaluation (CDE), NMPA, on January 15, 2024, Biosyngen’s third global exclusive product pipeline BRL03 was granted an IND approval for the treatment of advanced solid tumors, against lung cancer and gastric cancer.READ MORE -
 2023-10-27Biosyngen's BST02, the World's First TIL Therapy for Liver Cancer, is Granted an IND Approval by FDAOn October 26, 2023, Biosyngen’s TIL therapy BST02 for liver cancer was granted an approval for clinical trial by the US FDA. BST02, a breakthrough product in the field of cell and gene therapy, represents the world's first TIL therapy designed for the treatment of all types of liver cancer to progrREAD MORE
2023-10-27Biosyngen's BST02, the World's First TIL Therapy for Liver Cancer, is Granted an IND Approval by FDAOn October 26, 2023, Biosyngen’s TIL therapy BST02 for liver cancer was granted an approval for clinical trial by the US FDA. BST02, a breakthrough product in the field of cell and gene therapy, represents the world's first TIL therapy designed for the treatment of all types of liver cancer to progrREAD MORE -
 2023-09-18Biosyngen received the Asia Pacific Cell & Gene Therapy Excellence Awards (APCGTEA) 2023On September 14, 2023, Biosyngen Pte Ltd (hereinafter referred to as "Biosyngen") was the recipient of the prestigious "Most Promising Cell Therapy Pipeline in APAC" award at the 7th Annual Cell & Gene Therapy World Asia 2023, amidst a pool of international biotechnology companies.READ MORE
2023-09-18Biosyngen received the Asia Pacific Cell & Gene Therapy Excellence Awards (APCGTEA) 2023On September 14, 2023, Biosyngen Pte Ltd (hereinafter referred to as "Biosyngen") was the recipient of the prestigious "Most Promising Cell Therapy Pipeline in APAC" award at the 7th Annual Cell & Gene Therapy World Asia 2023, amidst a pool of international biotechnology companies.READ MORE -
 2023-09-11Biosyngen received FDA approval for Phase I/II Clinical Trials for BRL03, targeting Lung Cancer, Gastric Cancer and other advanced Solid TumorsOn September 9th, 2023, Biosyngen Pte Ltd (hereafter as “Biosyngen”) announced that the U.S. FDA has cleared the Investigational New Drug (IND) application for Phase I/II clinical trial of BRL03 for the treatment of lung cancer, gastric cancer and other advanced solid tumors. The approval granted toREAD MORE
2023-09-11Biosyngen received FDA approval for Phase I/II Clinical Trials for BRL03, targeting Lung Cancer, Gastric Cancer and other advanced Solid TumorsOn September 9th, 2023, Biosyngen Pte Ltd (hereafter as “Biosyngen”) announced that the U.S. FDA has cleared the Investigational New Drug (IND) application for Phase I/II clinical trial of BRL03 for the treatment of lung cancer, gastric cancer and other advanced solid tumors. The approval granted toREAD MORE -
 2023-06-05Biosyngen’s Cell Therapy BRG01 Granted Orphan Drug Designation by the U.S. FDA for Treatment of Nasopharyngeal CancerJun. 1st, 2023, Biosyngen Pte. Ltd. (hereinafter as “Biosyngen”) announced that the U.S. Food and Drug Administration’s (FDA) Office of Orphan Products Development (OOPD) has granted to its application, for immune cell therapy BRG01for the treatment of nasopharyngeal cancer, the status of Orphan DruREAD MORE
2023-06-05Biosyngen’s Cell Therapy BRG01 Granted Orphan Drug Designation by the U.S. FDA for Treatment of Nasopharyngeal CancerJun. 1st, 2023, Biosyngen Pte. Ltd. (hereinafter as “Biosyngen”) announced that the U.S. Food and Drug Administration’s (FDA) Office of Orphan Products Development (OOPD) has granted to its application, for immune cell therapy BRG01for the treatment of nasopharyngeal cancer, the status of Orphan DruREAD MORE



