Latest Headlines
CATEGORY
-
 2023-03-29PM Lee receives a first-hand update by Biosyngen on its progress in cell therapyThe city of Guangzhou welcomes Singapore Prime Minister Lee Hsien Loong, the first stop of his week-long visit to China. Accompanying PM Lee in this trip are Foreign Minister Vivian Balakrishnan, Trade and Industry Minister Gan Kim Yong, Health Minister Ong Ye Kung, Senior Minister of State for ForeREAD MORE
2023-03-29PM Lee receives a first-hand update by Biosyngen on its progress in cell therapyThe city of Guangzhou welcomes Singapore Prime Minister Lee Hsien Loong, the first stop of his week-long visit to China. Accompanying PM Lee in this trip are Foreign Minister Vivian Balakrishnan, Trade and Industry Minister Gan Kim Yong, Health Minister Ong Ye Kung, Senior Minister of State for ForeREAD MORE -
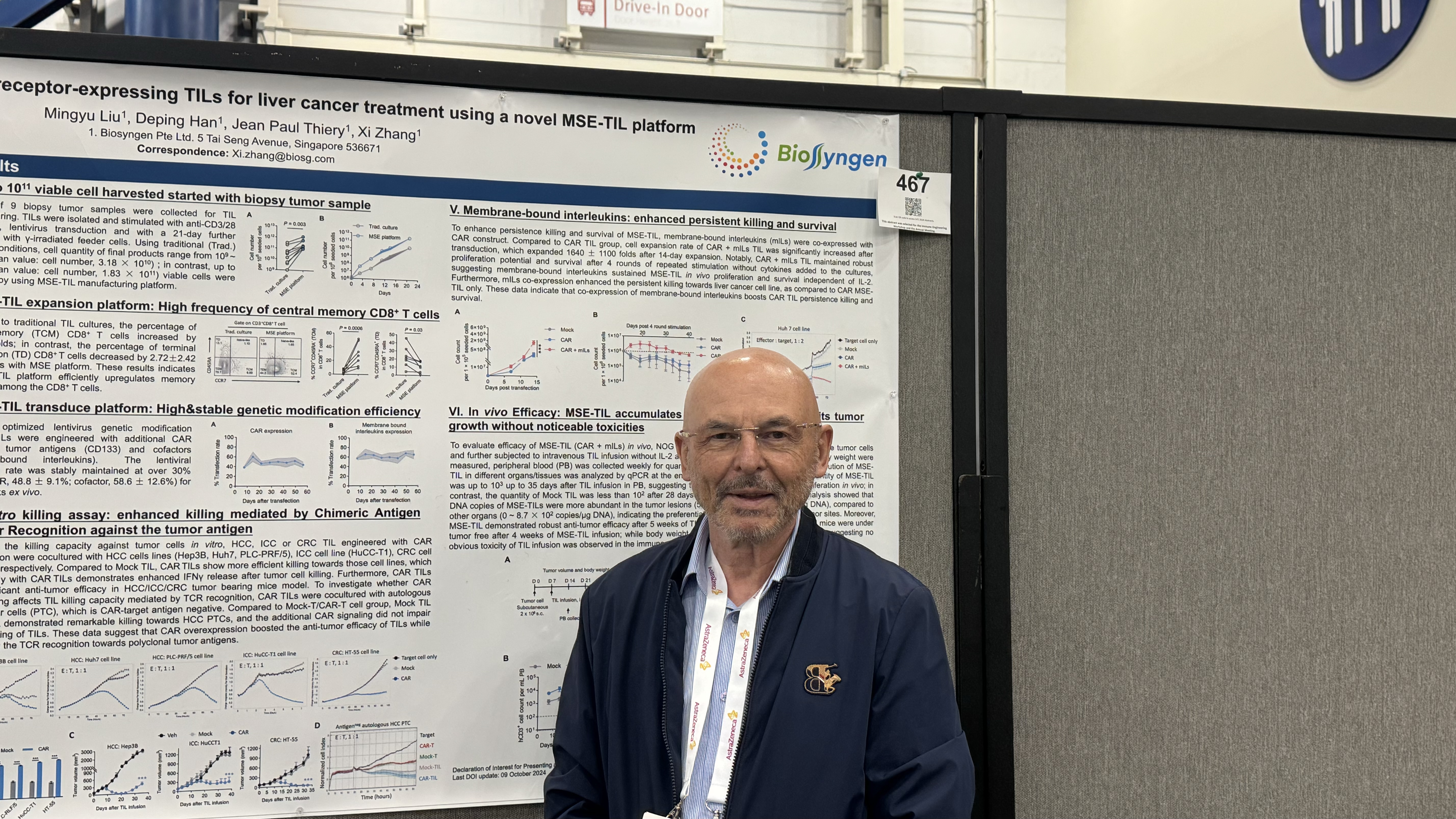 2024-11-13SITC 2024 (II): Biosyngen Unveils Next-Generation TIL Development Results – Achieving a Significant Breakthrough in the Treatment of All Types of Liver CancerFrom November 6 to 10, 2024, the highly anticipated 2024 Society for Immunotherapy of Cancer (SITC) Annual Meeting commenced in Houston, US. As one of the most influential annual events in the oncology field, this year's conference once again brings together top oncology experts and scholars from aREAD MORE
2024-11-13SITC 2024 (II): Biosyngen Unveils Next-Generation TIL Development Results – Achieving a Significant Breakthrough in the Treatment of All Types of Liver CancerFrom November 6 to 10, 2024, the highly anticipated 2024 Society for Immunotherapy of Cancer (SITC) Annual Meeting commenced in Houston, US. As one of the most influential annual events in the oncology field, this year's conference once again brings together top oncology experts and scholars from aREAD MORE -
 2024-11-13SITC 2024 (I): Historic Breakthrough in Cancer Treatment – Biosyngen’s Fourth-Generation Innovative Therapy Targeting Pan-Digestive Tract TumorsFrom November 6 to 10, 2024, the highly anticipated 2024 Society for Immunotherapy of Cancer (SITC) Annual Meeting commenced in Houston, US. As one of the most influential annual events in the oncology field, this year's conference once again brings together top oncology experts and scholars from aREAD MORE
2024-11-13SITC 2024 (I): Historic Breakthrough in Cancer Treatment – Biosyngen’s Fourth-Generation Innovative Therapy Targeting Pan-Digestive Tract TumorsFrom November 6 to 10, 2024, the highly anticipated 2024 Society for Immunotherapy of Cancer (SITC) Annual Meeting commenced in Houston, US. As one of the most influential annual events in the oncology field, this year's conference once again brings together top oncology experts and scholars from aREAD MORE -
2024-11-11Biosyngen Initiates Phase II Trial for BRG01, a Fourth-Generation Tumor Drug Addressing Targeted ToxicityOn November 10, 2024, Biosyngen held an investigator meeting in Guangzhou for the Phase II clinical study of BRG01 in treating relapsed/metastatic EBV-positive nasopharyngeal carcinoma. This hybrid meeting gathered leading experts and researchers in the field to discuss the clinical research protocoREAD MORE
-
 2024-09-19Biosyngen Presents Pioneering“Conditional Activation + Armor Enhancement” SUPER-T technology at ESMO 2024Barcelona, Spain – September 13-17, 2024 – The highly anticipated 2024 European Society for Medical Oncology (ESMO) Annual Congress has taken place in Barcelona, Spain. As one of the most influential annual gatherings in oncology, this congress brings together leading cancer experts and researchersREAD MORE
2024-09-19Biosyngen Presents Pioneering“Conditional Activation + Armor Enhancement” SUPER-T technology at ESMO 2024Barcelona, Spain – September 13-17, 2024 – The highly anticipated 2024 European Society for Medical Oncology (ESMO) Annual Congress has taken place in Barcelona, Spain. As one of the most influential annual gatherings in oncology, this congress brings together leading cancer experts and researchersREAD MORE -
 2024-09-19Biosyngen Best-in-Class Next-Generation Tumor-Infiltrating Lymphocyte (TIL) Technology Debuts on ESMO 2024 Annual MeetingBarcelona, Spain – September 13-17, 2024 – The highly anticipated 2024 European Society for Medical Oncology (ESMO) Annual Congress has taken place in Barcelona, Spain. As one of the most influential annual gatherings in oncology, this congress brings together leading cancer experts and researchersREAD MORE
2024-09-19Biosyngen Best-in-Class Next-Generation Tumor-Infiltrating Lymphocyte (TIL) Technology Debuts on ESMO 2024 Annual MeetingBarcelona, Spain – September 13-17, 2024 – The highly anticipated 2024 European Society for Medical Oncology (ESMO) Annual Congress has taken place in Barcelona, Spain. As one of the most influential annual gatherings in oncology, this congress brings together leading cancer experts and researchersREAD MORE -
 2024-09-18Biosyngen’s first-in-class CAR-T asset targeting solid tumors has entered phase II trial Phase I trial data debut at ESMO 2024 Annual CongressBarcelona, Spain – September 13-17, 2024 – The highly anticipated 2024 European Society for Medical Oncology (ESMO) Annual Congress has taken place in Barcelona, Spain. As one of the most influential annual gatherings in oncology, this congress brings together leading cancer experts and researchersREAD MORE
2024-09-18Biosyngen’s first-in-class CAR-T asset targeting solid tumors has entered phase II trial Phase I trial data debut at ESMO 2024 Annual CongressBarcelona, Spain – September 13-17, 2024 – The highly anticipated 2024 European Society for Medical Oncology (ESMO) Annual Congress has taken place in Barcelona, Spain. As one of the most influential annual gatherings in oncology, this congress brings together leading cancer experts and researchersREAD MORE -
 2024-08-13Biosyngen’s BRG01 Receives FDA Approval for Phase II Clinical TrialBiosyngen is proud to announce that the U.S. Food and Drug Administration (FDA) has approved its BRG01, an EBV-specific CAR-T cell therapy, to proceed with a pivotal Phase lI clinical trial. This marks the first cell therapy to enter Phase lI trials in both the U.S. and China for the treatment of reREAD MORE
2024-08-13Biosyngen’s BRG01 Receives FDA Approval for Phase II Clinical TrialBiosyngen is proud to announce that the U.S. Food and Drug Administration (FDA) has approved its BRG01, an EBV-specific CAR-T cell therapy, to proceed with a pivotal Phase lI clinical trial. This marks the first cell therapy to enter Phase lI trials in both the U.S. and China for the treatment of reREAD MORE -
 2024-08-01Biosyngen Partners with Singapore's Agency for Science, Technology and Research (A*STAR) to Advance Autoimmune TherapyBiosyngen has announced a strategic collaboration with Singapore's Agency for Science, Technology and Research (A*STAR) to enhance autoimmune therapy. The partnership was signed during the 14th Meeting of the Singapore-Guangdong Collaboration Council, held at China-Singapore Guangzhou Knowledge CityREAD MORE
2024-08-01Biosyngen Partners with Singapore's Agency for Science, Technology and Research (A*STAR) to Advance Autoimmune TherapyBiosyngen has announced a strategic collaboration with Singapore's Agency for Science, Technology and Research (A*STAR) to enhance autoimmune therapy. The partnership was signed during the 14th Meeting of the Singapore-Guangdong Collaboration Council, held at China-Singapore Guangzhou Knowledge CityREAD MORE -
 2024-07-15Biosyngen’s BRG01 enters Phase II clinical trial, a first-in-kind autologous EBV-Specific CAR-T Therapy for Solid Tumors on Recurrent/Metastatic Nasopharyngeal CarcinomaBiosyngen, a leading biotechnology company focused on the development of innovative cell therapies, recently announced that the Center for Drug Evaluation (CDE) of the National Medical Products Administration(NMPA) in China has approved the initiation of a pivotal Phase ll clinical trial evaluatingREAD MORE
2024-07-15Biosyngen’s BRG01 enters Phase II clinical trial, a first-in-kind autologous EBV-Specific CAR-T Therapy for Solid Tumors on Recurrent/Metastatic Nasopharyngeal CarcinomaBiosyngen, a leading biotechnology company focused on the development of innovative cell therapies, recently announced that the Center for Drug Evaluation (CDE) of the National Medical Products Administration(NMPA) in China has approved the initiation of a pivotal Phase ll clinical trial evaluatingREAD MORE



