SITC 2024 (II): Biosyngen Unveils Next-Generation TIL Development Results – Achieving a Significant Breakthrough in the Treatment of All Types of Liver Cancer

From November 6 to 10, 2024, the highly anticipated 2024 Society for Immunotherapy of Cancer (SITC) Annual Meeting commenced in Houston, US. As one of the most influential annual events in the oncology field, this year's conference once again brings together top oncology experts and scholars from around the world, showcasing the latest advancements and providing high-quality educational and networking opportunities for oncology researchers and healthcare professionals globally.
Biosyngen, an innovative biotechnology company specializing in immune cell therapies, presented its groundbreaking research on engineered CAR-TIL targeting liver cancer, developed using its proprietary MSE-TIL technology platform.
Abstract Title:
Engineered chimeric antigen receptor-expressing TILs for liver cancer treatment using a novel MSE-TIL platform
Abstract No.:
467
Tumor-infiltrating lymphocytes (TILs) are heterogeneous lymphocyte populations within the tumor microenvironment that contains T cells capable of recognizing tumor- or virus-associated antigens. In February, the FDA approved the first TIL-based therapy used with IL-2 for advanced metastatic/recurrent melanoma. However, due to the variability in T cell infiltration across different tumor types, differential abundance of antigen-specific T cells with robust immune response functionality, and the dependence of TIL anti-tumor efficacy on concomitant use of high-dose IL-2, the therapeutic applications of non-edited TILs are quite limited outside of melanoma.
To address this challenge, Biosyngen has developed the Multiple Signal Enhancing TIL (MSE-TIL) technology platform, featuring an efficient automated TIL production system and a robust in vitro genetic modification system. This platform improves TILs' in vivo expansion, long-term persistence, and tumor-killing efficacy.
By utilizing fresh or cryopreserved biopsy samples, we can expand TILs in vitro, achieving a final cell count of 10^11 in just four weeks while maintaining a high proportion of memory cells.
Our lentiviral vector platform allows for targeted genetic modifications of TILs, enhancing their specificity and cytotoxicity through the expression of various chimeric antigen receptors (CAR) tailored to different indications. Additionally, we improve the expansion and survival of CAR-TILs by co-expressing membrane-bound cytokines. These modifications are stably expressed for up to 60 days, with an average expression rate exceeding 50%.
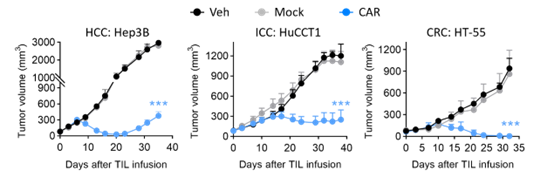
Figure 1: Antitumor Efficacy of CAR-TILs Developed Using the MSE-TIL Technology Platform Against Multiple Tumors
In subcutaneous tumor models involving various cancers such as hepatocellular carcinoma, intrahepatic cholangiocarcinoma, and colorectal cancer (Figure 1), CAR-TILs demonstrated significant antitumor efficacy compared to Mock TILs (without IL-2 co-injection). Moreover, CAR-TILs co-expressing membrane-bound cytokines (CAR + mILs, Figure 2) exhibited enhanced in vivo survival capabilities, resulting in sustained antitumor efficacy and prolonged tumor-free survival in mice (without IL-2 co-injection), without causing any adverse effects (no weight loss observed).
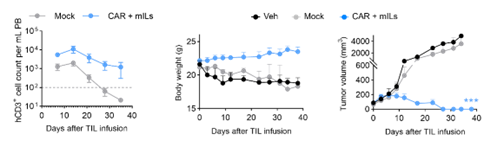
Figure 2: Enhanced Antitumor Efficacy of CAR-TILs in Liver Cancer-Bearing Mice
Biosyngen's BST02, developed from the MSE-TIL platform for treating advanced liver cancer (all types), is currently in a Phase I clinical trial. The low-dose cohort has been successfully enrolled and infused, showing promising safety and efficacy with over patient achieving a 60% partial response (PR) 48 days post-infusion. Recruitment for all Phase I participants is expected to be completed by the end of this year.
About Biosyngen’s Innovative MSE-TIL Technology Platform
- High-Efficiency Automated TIL Production System: This platform allows for TIL preparation using tumor biopsy samples. Both tumor tissue and final products can be cryopreserved, overcoming geographical limitations.
- Efficient In Vitro Genetic Engineering System: Utilizing viral vector technology, our system achieves stable genetic modification of TILs, maintaining a transfection efficiency of over 50%.
- Effective In Vivo Expansion and Long-Term Persistence: The proportion of central memory T cells (TCM) in the final TIL product is increased by more than eightfold.
- Robust Tumor Cytotoxicity: The TILs exhibit sustained anti-tumor activity without the need for concurrent interleukin-2 administration.
Figure 3. Biosyngen’s MSE-TIL technology platform
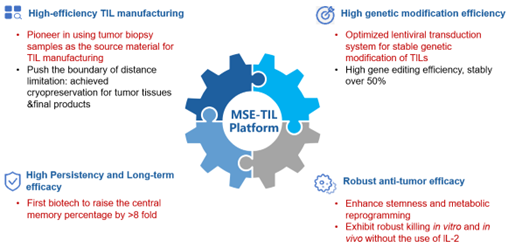
Figure 3. Biosyngen’s MSE-TIL technology platform


