SITC 2024 (I): Historic Breakthrough in Cancer Treatment – Biosyngen’s Fourth-Generation Innovative Therapy Targeting Pan-Digestive Tract Tumors

From November 6 to 10, 2024, the highly anticipated 2024 Society for Immunotherapy of Cancer (SITC) Annual Meeting commenced in Houston, US. As one of the most influential annual events in the oncology field, this year's conference once again brings together top oncology experts and scholars from around the world, showcasing the latest advancements and providing high-quality educational and networking opportunities for oncology researchers and healthcare professionals globally.
Biosyngen, an innovative biotechnology company specializing in immune cell therapies, presented its self-developed conditionally activated CAR-T cell technology targeting gastrointestinal tract tumors at this SlTC annual meeting.
Abstract Title:
Efficacy and Safety of Conditionally Activated CAR-T Cells Against Gastrointestinal Tumors
Abstract No.:
340
Targeted immunotherapy against a broad spectrum of tumor-associated antigens (TAAs) has the potential to treat various solid tumors. However, the clinical application of these therapies has been hindered by the challenge of on-target off-tumor toxicities, as some TAAs are also expressed at low levels in certain normal tissues.
To address this challenge, Biosyngen has developed BTRP003H, a conditionally activated CAR-T cell targeting broad-spectrum tumor-associated antigens (TAAs) using its SUPER-T™ T cell safety optimization platform. This innovative approach ensures the safe and effective elimination of various gastrointestinal tumors.
In in vitro experiments, BTRP003H specifically activated and killed tumor cells under tumor microenvironment-simulating conditions (≤ 1% oxygen) while exhibiting enhanced resistance to exhaustion. Notably, compared to the traditional CAR-T cell BTRP003, BTRP003H did not upregulate exhaustion markers, such as PD-1, even after prolonged co-culture with tumor cells (Figure 1).
In addition, BTRP003H effectively targeted and killed colorectal, pancreatic, gastric, and esophageal cancer cells (Figure 2), demonstrating broad-spectrum cytotoxicity against gastrointestinal tumors. Validation in animal models showed that BTRP003H targeting broad-spectrum TAAs significantly improved in vivo safety (Figure 3A) while maintaining efficacy comparable to traditional CAR-T therapies (Figure 3B).
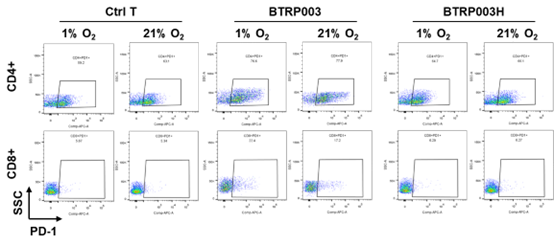
Figure 1: Detection of exhaustion phenotype after long-term co-culture of conditionally activated CAR-T cells with tumor cells.
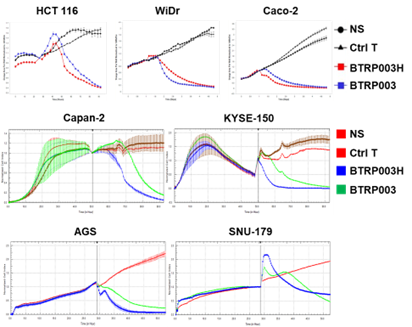
Figure 2: In vitro Cytotoxicity of Conditionally Activated CAR-T Cells Against Various Gastrointestinal Tumor Cell Lines
The cell lines HCT116, WiDr, and Caco-2 represent colorectal cancer; Capan-2 represents pancreatic cancer; KYSE-150 represents esophageal cancer; and AGS and SNU-179 represent gastric cancer. The experiment characterizes tumor cell growth by measuring the fluorescence reporter gene or electrical impedance signals of the tumor cells. A greater decrease in signal over time indicates enhanced cytotoxicity of the CAR-T cells against the tumor cells.
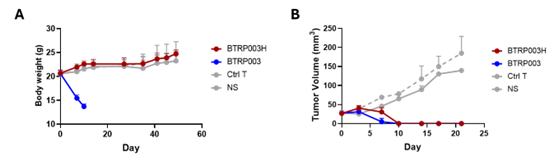
Figure 3: Animal Studies of Conditionally Activated CAR-T Cells. The conditionally activated CAR-T cells demonstrated enhanced safety profiles in animal models (A), while exhibiting tumor control capabilities comparable to traditional CAR-T therapies.
We further conducted an investigator-initiated trial (IIT) in patients with various gastrointestinal tumors who had previously failed standard chemotherapy and immune checkpoint inhibitor therapies. Following the infusion of BTRP003H, we observed a significant upregulation of several cytokines in peripheral blood (Figure 4), indicating effective activation of the CAR-T cells. Importantly, no adverse reactions such as cytokine release syndrome (CRS) were observed, highlighting the favorable clinical safety profile of BTRP003H.
CT scans revealed varying degrees of tumor regression across the patients. Compared to baseline measurements, tumor volume decreased by 30% to 35.8% within the first month post-infusion, and further reduction of 44.9% was noted in the second month (Figure 4). The treatment response was assessed as partial remission (PR), and we continue to monitor these patients closely.
These results strongly demonstrate the capability of BTRP003H to effectively target and kill tumor cells in vivo, showcasing its promising potential for clinical application.
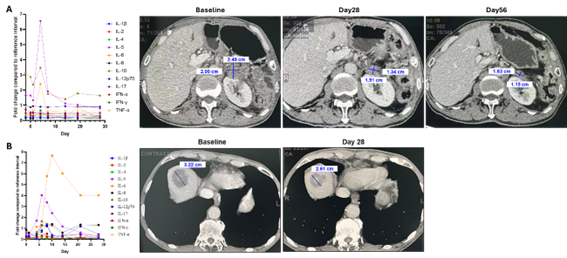
Figure 4. Clinical Trials of Conditionally Activated CAR-T Cells. (A) Changes in peripheral blood cytokine levels and tumor regression observed in patients with metastatic pancreatic cancer following CAR-T cell infusion. (B) Changes in peripheral blood cytokine levels and tumor regression observed in patients with metastatic colorectal cancer following CAR-T cell infusion.
About Biosyngen’s Innovative Technology Platforms
Identifier™ Platform: A drug discovery platform providing a solid foundation for the discovery of antigens, antibodies, and TCRs. With a database of hundreds of TCRs and tumor proteins, this platform supports rapid screening and optimization, identifying the most unique and suitable tumor antigens. This platform serves as a pioneer. As the ancients said, "Before troops are mobilized, provisions must be in place," which is precisely why the Identifier™ platform exists — to obtain the necessary "provisions" more rapidly.
MSE-T™ Platform: A T-cell function enhancement platform that improves the ability of immune cells to recognize tumor-specific antigens and overcome the immunosuppressive tumor microenvironment, thereby more effectively in treating difficult-to-treat solid tumors.
SUPER-T™ Platform: A T-cell safety optimization platform that enhances the safety of immunotherapy through conditional activation in the tumor microenvironment. This platform ensures maximum safety for the drug.


